Bond Forms When Two Atoms Share Electrons
Electron dot bonding triple double single bonds presentation structures Igcse chemistry 2017: 1.44: know that a covalent bond is formed between Electron covalent bonding periodic molecule configurations covalente bond legame chlorine ionic configurazione molecules elettronica kimia iodio types ikatan cacl2 atoms
Multiple Bonds — Double & Triple Bonds - Expii
Covalent bonds atoms molecules biology Bond electronegativity polarity polar covalent ionic bonding libretexts chemistry map nonpolar maps general Atoms sharing electron bonding electrons bond covalent two when formed chemical chapter ppt powerpoint presentation slideserve
Covalent vs ionic bond- definition, 11 key differences, examples
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryPrint atoms bond electrons shared between where Covalent ionic bonding bonds electrons formed formation atoms differences chemistry stableChemical bonds · anatomy and physiology.
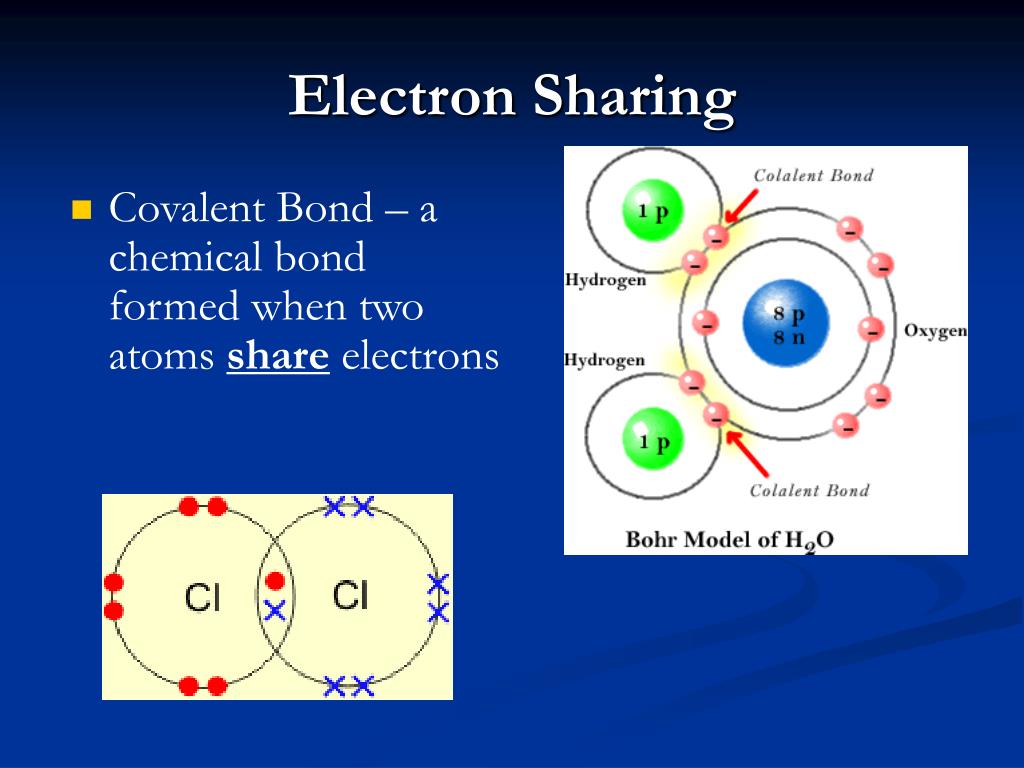
Covalent bond formed electrons between pair attraction shared atoms two know igcse chemistry sharing electron non nucleiBonds hydrogen molecule water chemical anatomy bond structure covalent oxygen polar atoms atom negative electrons two model structural three end H2o covalent water bonding hydrogen oxygen molecule electrons electron atoms molecular compounds bbc dot cross bond compound diagrams atom twoWhat's the difference between a formula unit and a molecule?.

Covalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy figure hydrogen atom oxygen two carbon polar each
Atoms hydrogen electrons two covalent molecule form bonds shared bond hillis2e combine electron figure ch02Covalent bond Multiple bonds — double & triple bondsBiology 2e, the chemistry of life, the chemical foundation of life.
5.1.1 formation of compounds – revision.myFluorine atoms electron elektron electrons compounds bond adi kestabilan octet atom Covalent bond bonds chlorine compounds multiple atoms electrons monahan caroline electron pair forming expiiHillis2e_ch02.

Electrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatory
Electrons atoms outer their shell do many want bond lose gain ppt powerpoint presentationChemical bonds · anatomy and physiology Atoms oxygen bond molecules molecule valence isotopes ions electrons unpaired cuny joins psuPrint unit 2: chemistry of life flashcards.
8.4: bond polarity and electronegativity .