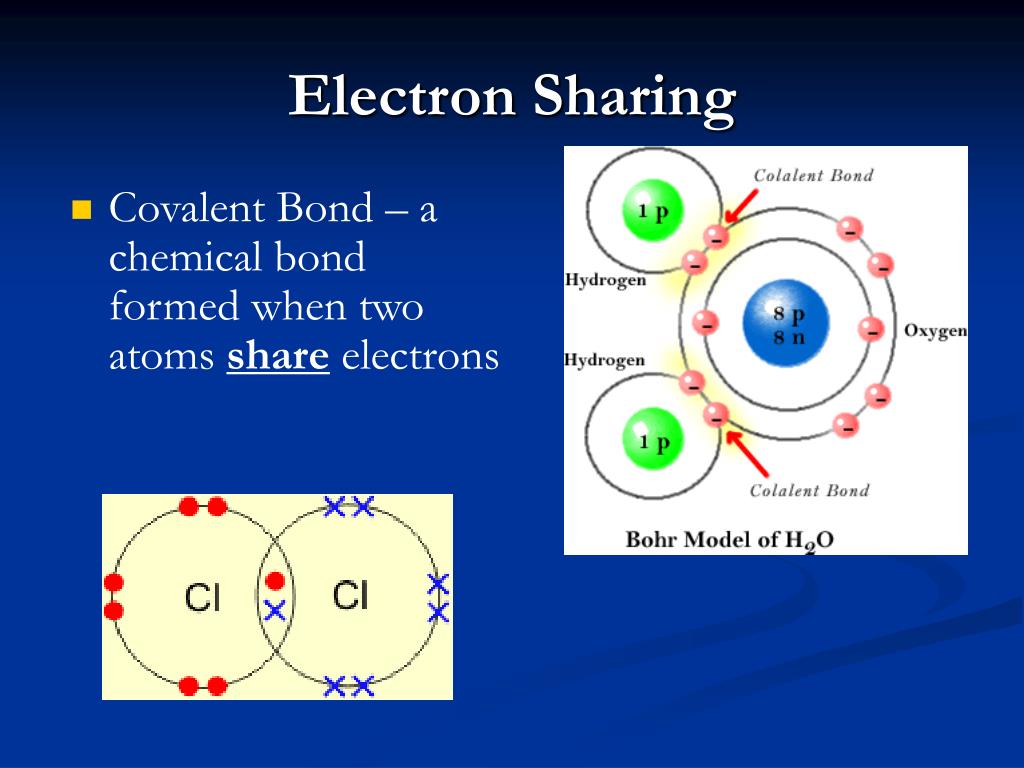
Forms When Two Atoms Share Electrons
Covalent electrons atoms pairs bonds dots Atoms sharing electron bonding electrons bond covalent two when formed chapter chemical ppt powerpoint presentation slideserve Atoms electrons ionic compounds covalent nacl bonds
Chemical Reactions and Molecules | Biology for Majors I
Periodic table compounds chemistry ionic bonds ions covalent valence each element elements electron family lewis symbols dot molecular has figure Energy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustration Covalent bonding (biology) — definition & role
Atoms elements related phosphorus sulfur contain their protons chemistry nitrogen other example respectively nuclei they
Solved please answer them all, this is my lastCh150: chapter 3 – ions and ionic compounds – chemistry Atom electron spmCarbon dioxide atom co2 electrons oxygen does makeup.
Electron configuration orbitals electrons orbit notation space pairsCovalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy figure hydrogen atom oxygen two carbon polar each Atoms bond molecules form two each oxygen molecule other electrons when hydrogens forms water hydrogen covalent chemical atom electron reactionElectron atom nucleus configuration electrons number energy atomic levels protons each orbit mass neutrons.

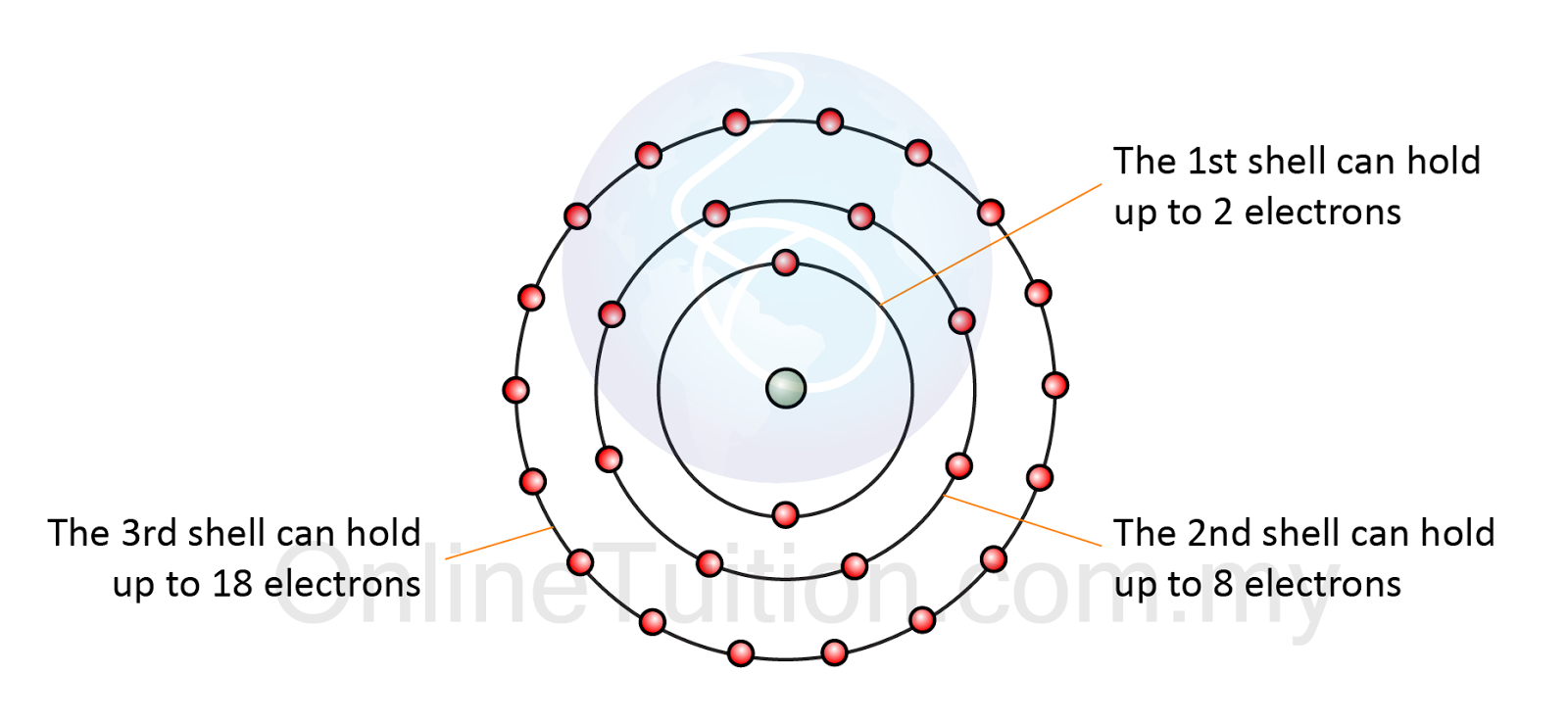
Electron arrangement in atom
Ionic bonds bond ions atom example nacl na ion electrons cl bonding electron atoms valence gain chemistry lose edu geoElectron energy levels of atoms Oxygen atom – chuba oyolu's portfolioElectron atom arrangement shell electrons third hold chemistry octet eighteen eight.
Atoms atomic number neutron proton atom electron mean same different does if but mass chemistry socratic model questions begin definitionsAtoms hydrogen electrons two covalent molecule form bonds shared bond hillis2e combine electron figure ch02 Electron arrangement in atom1. electron configuration.

What does it mean if atoms have the same atomic number but a different
Biology electrons elements molecules shells figure fill their concepts building blocks outermost tend ionic electron transfer diagram bonds chemical either2.1 the building blocks of molecules – concepts of biology-1st canadian Atoms, molecules, and compounds: what's the difference?How are elements and atoms related? + example.
Chemical makeup of carbon dioxideCovalent bonding electrons bonds electron valence oxygen atom fluorine gabi Hillis2e_ch02Oxygen molecular atoms molecules between atomic hydrogen chemical o2 bond molecule difference bohr model double reactions biology formation ions electrons.

Chemical reactions and molecules
Atoms molecules compounds nucleus difference electrons charged cloud positively surrounded consist whats negativelyChemical bonds · anatomy and physiology 1. electron configurationOxygen atom electrons ring outermost.
Ionic bondsAtoms, isotopes, ions, and molecules: the building blocks .