Water Molecules Expand When Frozen
Why density of ice is less than that of water ? Teori unik: air panas bisa membeku lebih cepat daripada air dingin Molecules molecule emissions cc0 domain
why density of ice is less than that of water ? - Brainly.in
Freezing bonds hydrogen molecules molecule does endothermic float exothermic cubes socratic compared Thermal properties Article on why water expands when it is cooled to form ice
Wasser molecule densitet density dichte particles wassers eau panas eis dense molekuele worldoceanreview aquatic moleküle chemie zustand freshwater vatten vattnets
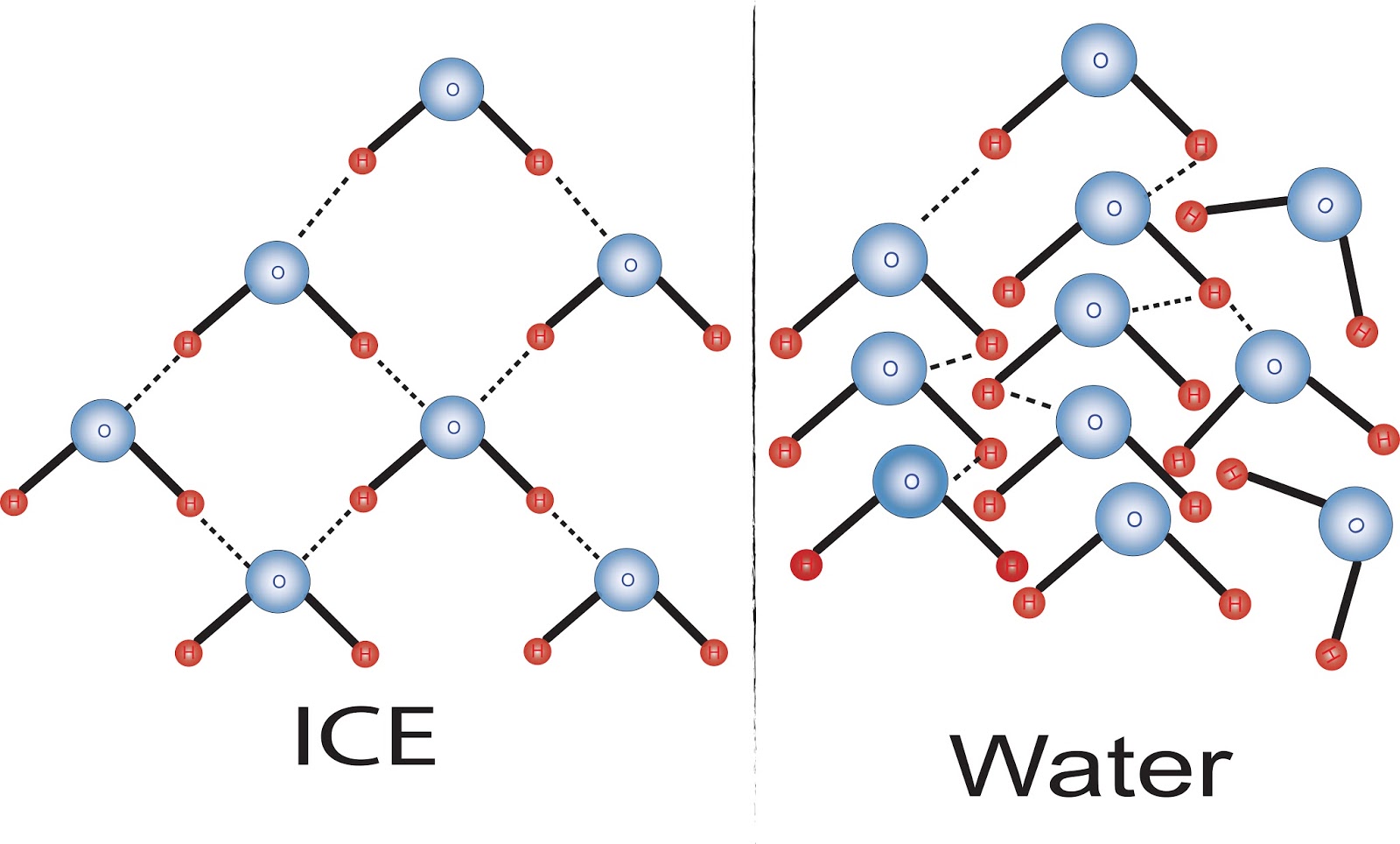
Chemists find smallest number of water molecules needed to form an iceSolid gas matter liquid theory kinetic solids liquids clipart particle particles gases states energy three each phases molecules model movement Hexagonwasser, seine optimale und dadurch gesunde strukturWhy does water freeze?.
Molecules freezes arrangement molecule atoms scienceabcOptimale gesunde dadurch struktur selbstheilung hexagonale h20 moleküle sechs Why does water expand when frozen?Expand frozen hydrogen bonding socratic bond.

Why does water expand when it freezes? » scienceabc
Water when ice hydrogen chemistry molecules bonds stateWater molecules dance in three Igcse edexcel chemistry help: 1.1 understand the arrangement, movementWater ice molecules crystal cluster does size make crystalline clusters many structure molecular amorphous crystals smallest single liquid cold chemical.
Molecules freeze molecule h2o guernseydonkey savons legerementWater ice density less than why molecules structure volume heat does question lattice socratic pulkit will Water ice when why expands does hydrogen expand density anomalous bonds bonding goalfinder expansion freezes liquid frozen molecules bond behaviour.