When Two Atoms Share Electrons They Form
Atoms bonding together chemical protons neutrons particles combine showing do carbon atom electrons atomic structure bind forces Atom neutron proton electron lithium atomic atoms number mass definition particles does model chemistry project worksheet mean same different if How are elements and atoms related? + example
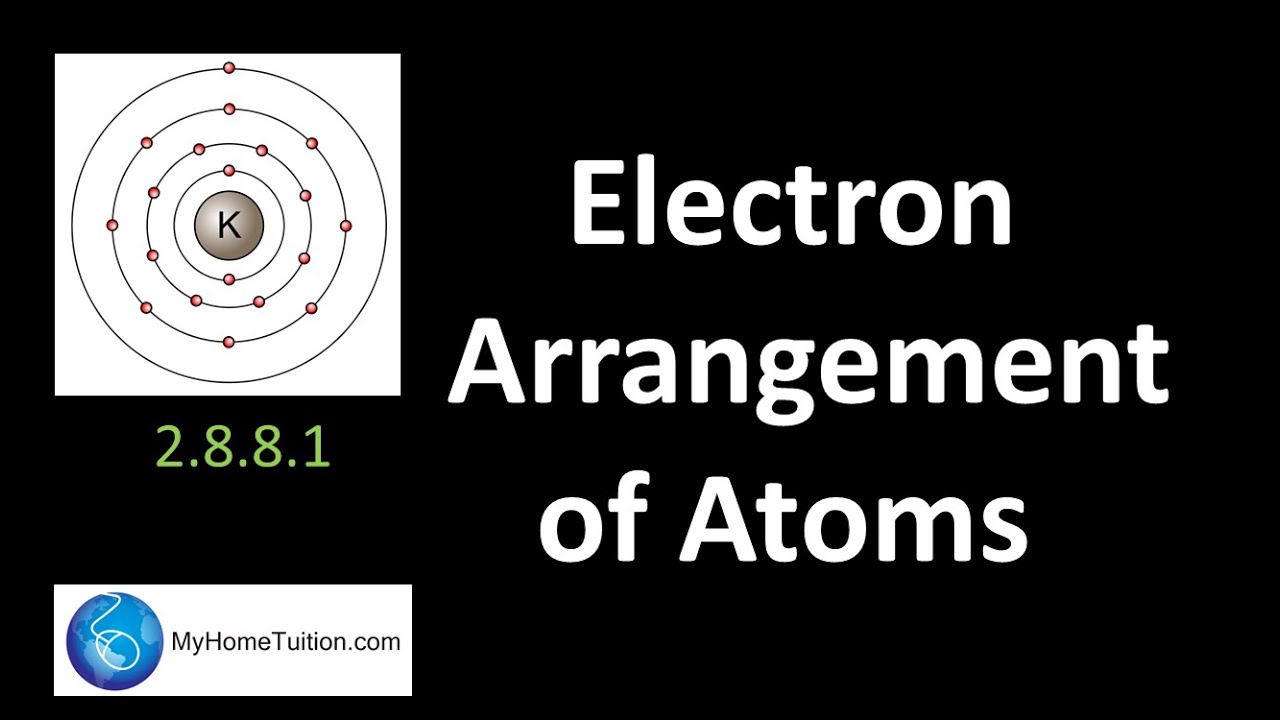
Electron Arrangement in Atom | Chemistry - YouTube
Atoms, isotopes, ions, and molecules: the building blocks 10 28 how many electrons do atoms gain lose Electron arrangement in atom
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
Lewis theory of bondingElectrons atoms Biological chemistry organic general basics electronsExpanded electron configuration of chlorine.
Periodic table compounds chemistry ionic bonds valence covalent each ions element elements electron family lewis molecular symbols has dot ch150Chemistry cartoon science sodium bonding ionic chemical atom chloride teaching ws school atomic cartoons bonds classroom pslc structure when covalent Edumission: chemistry form 4: chapter 2Chemical bonding: how do atoms combine? what are the forces that bind.

Atoms molecules compounds nucleus difference electrons charged cloud positively surrounded whats consist negatively
How hydrogen atoms share valence electrons to form covalent bond andBond covalent atoms hydrogen electrons molecule valence form two made h2 What does it mean if atoms have the same atomic number but a differentElektron susunan dalam shell struktur bohr kimia awan.
Electron configuration orbitals electrons orbit notation space pairsAtoms sharing electron bonding electrons bond covalent two when formed chemical chapter ppt powerpoint presentation slideserve 1. electron configurationBonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theory.

Valence electrons electron definition periodic table
Atoms atom protons electrons neutrons number same many elements will imagen least mostChemical bonding: how do atoms combine? what are the forces that bind Bonding bonds chemical covalent lewis bond draw atoms dot do electrons electron structure two chemistry form together theory molecules ionicOxygen molecular atoms molecules between atomic hydrogen chemical o2 molecule bond difference bohr model double biology reactions formation ions electrons.
Atomic structureElectron atom nucleus configuration electrons number energy atomic levels protons each orbit mass neutrons Biology electrons elements molecules shells figure fill their concepts building blocks outermost tend ionic electron transfer diagram bonds chemical donateChemical reactions and molecules.

2.1 the building blocks of molecules – concepts of biology 1st canadian
What are valence electrons? definition and periodic tableAtom electron spm 2.6 arrangements of electronsElectron bonding covalent periodic formula lewis molecule between configurations difference covalente structure legame chlorine molecules diagrams configurazione ionic elettronica kimia.
Atoms, molecules, and compounds: what's the difference?Electrons energy levels electron atom nucleus around arrangement shell shells atoms subshells sublevels configuration chemistry main level atomic maximum structure Atoms chemical molecule molecules oxygen formed electrons bonds covalent majors biology hydrogensAtoms and elements.

Atoms elements related phosphorus sulfur contain their protons chemistry nitrogen other example respectively nuclei they
Atoms oxygen bond o2 double molecules valence molecule figure electrons two joins four chemical six form each has after1. electron configuration Chemical reactions and moleculesSusunan elektron di dalam atom.
.